
THE CHEMISTRY OF LIFE
Hydrogen Bonding
Overview: A hydrogen bond is a weak force occurring when a hydrogen atom (with a positive dipole) is bonded to a strongly electronegative atom (which will have a negative dipole). An atom such as this will form a weak attraction with other electronegative atoms with similar dipole movements. The bonds are generally stronger than regular dipole-dipole bonds, but weaker than covalent and ionic bonds.
Importance of hydrogen bonding:
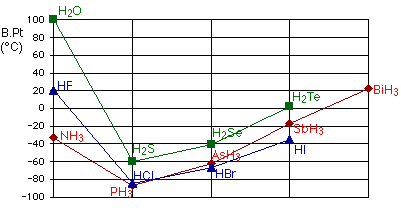
The graph below is a graph of the group four (carbon group) molecules’ boiling point when fully bonded with hydrogens. In nonpolar molecules such as these, dipole-dipole movements are the main source of intermolecular forces, so as the size of a molecule increases, so too do the intermolecular forces between molecules.

However, as we go across period two, something unusual happens. Water has a much higher boiling point than both ammonia and hydrogen fluoride, and these have higher boiling points than other element and hydrogen combinations in their groups. It seems extremely strange until we consider exactly how hydrogen bonding works.
There are three main molecules with this type of bonding: N, O, and F. Each of these, when fully saturated with hydrogen molecules will have one or more lone pairs.
It should make sense at this point that the highly electronegative Oxygen in H2O will unevenly share electrons with Hydrogen.

What this means, is that hydrogen combined with an electronegative atom will create what is called a permanent dipole. A permanent dipole in water involves the Oxygen “hogging” the electrons, resulting in positive hydrogens and a very negative oxygen.
http://www.chemguide.co.uk/atoms/bonding/electroneg.html#top
For more information on electronegativity
We already understand that opposite charges attract, and by now you should already see where this is going. A hydrogen bond occurs when a positive hydrogen bonded to an N, O, or F is attracted to the negative dipole of a lone pair of another molecule.
A nonpolar molecule will never form hydrogen bonds because there is no negative dipole for a hydrogen to be attracted to.
Curious minds:
Those who are more inquisitive may be wondering why hydrogen bonding only seems to happen for N, O, and F, and not for larger molecules with similar electronegativity. The answer? The lone pairs of the smaller atoms are less diffuse, and have a greater charge density than say... Sulfur or Chlorine. In larger molecules, the lower concentration
of electrons and corresponding decrease in charge density means that electrons are no longer as attracted to positive charges.
Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are being constantly broken and reformed in liquid water.

Donors and Acceptors
In order for a hydrogen bond to occur we need both an acceptor and a donor for the electrons. The acceptor in this case is the Hydrogen, and the donor is the highly electronegative electron pair on an N, O, or F atom. The large discrepancy in charge leads to a mutual attraction which leads to vast intermolecular attractions.
How Strong are Hydrogen Bonds, really?:
Again, Hydrogen bonds are about 1/10 the strength of Covalent bonds. But how strong are those? Honestly, it depends what we're looking at.
Intramolecular:
Covalent > Ionic > Hydrogen > Hydrophobic > van der Waals
Intermolecular
Ionic > Covalent > Hydrogen > Hydrophobic > van der Waals
Intermolecular (aqueous)
Covalent > Hydrogen > Ionic > Hydrophobic > van der Waals
On what the different bond types look like
This is a lot to wrap your head around at first, so let's break it down a bit.
Intramolecular forces are forces within a molecule.
Knowing this, it is intuitive that there are going to be fewer hydrogen bonds than covalent and ionic bonds. After all, you need a large molecule to allow an electron pair near a positive hydrogen, compared to the covalent and ionic bonds you will find everywhere.
Hydrophobic forces and Van Der Waals forces are very weak, and will always be the weakest forces when the others exist.
Curious minds:
Why do Hydrogen bonds and Covalent bonds suddenly overtake Ionic bonds in an aqueous solution? The answer is suprisingly simple! In water, ionic things will dissociate into ions! We've prepared a video to aid your understanding.
Now where do we find these types of bonds? Before we finish we'll give you some nifty examples of these bonds in a human body.
Covalent Bonds - polysaccharides, polypeptides.
Hydrogen - DNA base pairs are held together by these bonds.
Ionic - An amino group or carboxyl group that hasn't formed peptide bonds can form weak ionic bonds (The body is aqueous, so we'll only see weak Ionic bonds).
Hydrophobic - Found in the phospholipid membrane and create a semi-permeable membrane.
Van der Waals - Happen between all molecules to some extent, but for consistancy we'll say that they also happen between cells.